Free Case Evaluation
You will never be charged a fee unless a recovery is made for you.
We are no longer accepting new cases.
Tenofovir lawsuits are being filed by people who have taken Gilead’s HIV drug tenofovir disoproxil fumarate (TDF) and have been diagnosed with kidney disease and/or bone density loss. The TDF lawsuits claim that Gilead knew that their TDF drugs could harm users but elected to withhold a less toxic and arguably more effective version of the drug (tenofovir alafenamide fumarate or “TAF”) in order to maximize the profits from its line of tenofovir drugs.
If you or a loved one took TDF and have been diagnosed with either kidney disease or bone density loss (osteopenia or osteoporosis) contact us for a free consultation.
At Trustwell Law, our experienced attorneys take a personalized, compassionate approach. We cut through the legalese and partner with our clients. We have access to the expertise, resources, and manpower to fully investigate each case and fight for and with our clients to get the justice they deserve.
If you are looking for a TDF attorney, call us at 800-796-1636 or submit your case details online and someone will contact you shortly. You pay nothing unless your lawsuit is successful and you receive compensation.
Gilead’s HIV drug tenofovir disoproxil fumarate (TDF) is one of a class of antiretroviral drugs known as nucleoside reverse transcriptase inhibitors. (The HIV virus is a “retrovirus”—meaning RNA (not DNA) is its genetic material—so drugs to fight HIV are classified as “antiretroviral” drugs.) Reverse transcriptase is an enzyme crucial to the replication and progression of HIV. Nucleoside reverse transcriptase inhibitors like TDF do exactly what their name suggests: they impede, or slow, the activity of reverse transcriptase.
When used with other medicines, TDF drugs can slow the progression of HIV, keeping HIV-positive people healthy for years according to the Centers for Disease Control and Prevention (CDC).
HIV-positive people must take antiretroviral drugs. Not taking those drugs is not an option, regardless of their side effects.
There are five Gilead TDF drugs available to people with HIV:
TDF lawsuits allege that Gilead delayed developing and introducing a less-toxic antiretroviral drug with fewer side effects (TAF), thereby exposing HIV-positive patients to the TDF drugs with their highly detrimental side effects for years longer than necessary.
Here is an illuminating timeline of Gilead’s drug development/marketing of these drugs:
The lawsuit against Gilead claims that people who took Gilead’s TDF drugs were subjected to their extra toxicity for years due to Gilead delaying their work on the safer alternative. It appears Gilead stopped working on the safer TAF alternative in 2004 to maximize their profits from TDF before making another, safer drug available to the patients who have no choice but to take antiretrovirals.
Patients taking Gilead’s TDF drugs have an increased risk for kidney failure. Kidneys are vital organs. They filter our blood, sending waste out in the form of urine.
Kidneys are resilient organs; when damaged they adapt to keep working. So, by the time a person with kidney disease experiences issues and discovers the damage, it is often permanent and irreversible. In the worst cases, people with kidney failure need either dialysis, which involves a machine cleaning their blood, or a kidney transplant.
Symptoms and consequences of kidney failure include:
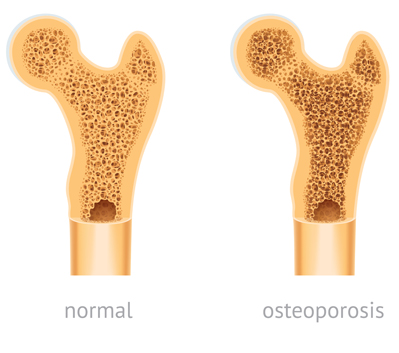
Several studies have found a correlation between TDF use and bone density loss. Healthy bone tissue never stops growing. New tissue grows to replace older tissue. But when the old tissue deteriorates faster than the new tissue can grow, or when new tissue stops growing completely, osteoporosis can develop. Osteoporosis is a bone condition where bones are so weak that minor pressure or what would normally be an insignificant fall can cause them to break.
Osteopenia is a condition that precedes osteoporosis. Many HIV patients who have taken TDF drugs claim they have developed osteopenia or osteoporosis.
Common symptoms of osteoporosis and osteopenia include:
If you or a loved one have taken any of Gilead’s TDF drugs (Atripla, Complera, Stribild, Truvada, or Viread) and have since been diagnosed with kidney problems or bone density loss, contact us. You may be eligible for compensation.
Article Sources
AIDS Healthcare Foundation. (2016, May 31). AHF Calls on FDA, Congress to Investigate Gilead Sciences Over HIV/AIDS Drug Patent Manipulation. Retrieved from https://www.aidshealth.org/2016/05/ahf-calls-fda-congress-investigate-gilead-sciences-hivaids-drug-patent-manipulation-ahf-media-availability/
AIDS Healthcare Foundation. (2016, June 7). AHF Launches “Gilead Scandal: Gay Men, We Don’t Care About Your Kidneys and Bones” – New National Ad Campaign. Retrieved from https://www.aidshealth.org/2016/06/ahf-launches-gilead-scandal-gay-men-dont-care-kidneys-bones-new-national-ad-campaign/
AIDS Healthcare Foundation. (2019, February 13). Victory over Gilead! California Court Rules HIV Drug Personal Injury Cases May Proceed. Retrieved from https://apnews.com/4db956edccff42d7b869cf311b3e5b0d
Centers for Disease Control and Prevention. (2020, May 18). Preventing New HIV Infections. Retrieved from https://www.cdc.gov/hiv/clinicians/prevention/prep.html
Grant, P. and A. Cotter. (2016, May). Tenofovir and Bone Health. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4844450/
Grigsby, I., et al. (2010, February 2). Tenofovir-associated bone density loss. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2817787/
Hirsch, J. (2019, July 30). Osteopenia and Osteoporosis: Is There a Difference? Retrieved from https://www.spineuniverse.com/conditions/osteoporosis/osteopenia-osteoporosis-there-difference
Mayo Clinic. (2019, August 15). Chronic kidney disease. Retrieved from https://www.mayoclinic.org/diseases-conditions/chronic-kidney-disease/symptoms-causes/syc-20354521
McComsey, G.A., et al. (2018, February 20). Switch from tenofovir disoproxil fumarate combination to dolutegravir with rilpivirine improves parameters of bone health. Retrieved from https://journals.lww.com/aidsonline/Fulltext/2018/02200/Switch_from_tenofovir_disoproxil_fumarate.8.aspx
Naisha v. Gilead Sciences. (2019, July 11). Complaint Under Delaware Superior Court Civil Rule 17 for Damages. Retrieved from https://aboutlawsuits-wpengine.netdna-ssl.com/wp-content/uploads/2019-7-11-truvada-naisha-complaint.pdf
Petersen, M. (2016, May 29). A history of Gilead’s biggest HIV drug. Retrieved from https://www.latimes.com/business/la-fi-gilead-timeline-20160527-snap-story.html
Petersen, M. (2016, May 29). AIDS Healthcare Foundation lawsuit vs. Gilead. Retrieved from https://documents.latimes.com/aids-healthcare-foundation-lawsuit-vs-gilead/
Petersen, M. (2016, May 29). A question of timing: A lawsuit claims Gilead Sciences could have developed a less-harmful version of its HIV treatment sooner. Retrieved from https://www.latimes.com/business/la-fi-gilead-20160529-snap-story.html
Petersen, M. (2018, May 9). Patients sue Gilead, saying drug company intentionally delayed safer HIV medicine. Retrieved from https://www.latimes.com/business/la-fi-gilead-hiv-drug-lawsuit-20180509-story.html
Silverman, E. (2016, February 1). Gilead accused of manipulating HIV patents. Retrieved from https://www.statnews.com/pharmalot/2016/02/01/gilead-patents-hiv/
Venter, W.D.F., J. Fabian, and C. Feldman. (2018, July 17). An overview of tenofovir and renal disease for the HIV-treating clinician. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6111387/
You will never be charged a fee unless a recovery is made for you.